Solid solution

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
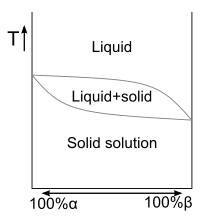
Fig. 1 A binary phase diagram displaying solid solutions over the full range of relative concentrations.
.mw-parser-output .quoteboxbackground-color:#F9F9F9;border:1px solid #aaa;box-sizing:border-box;padding:10px;font-size:88%;max-width:100%.mw-parser-output .quotebox.floatleftmargin:0.5em 1.4em 0.8em 0.mw-parser-output .quotebox.floatrightmargin:0.5em 0 0.8em 1.4em.mw-parser-output .quotebox.centeredmargin:0.5em auto 0.8em auto.mw-parser-output .quotebox.floatleft p,.mw-parser-output .quotebox.floatright pfont-style:inherit.mw-parser-output .quotebox-titlebackground-color:#F9F9F9;text-align:center;font-size:larger;font-weight:bold.mw-parser-output .quotebox-quote.quoted:beforefont-family:"Times New Roman",serif;font-weight:bold;font-size:large;color:gray;content:" “ ";vertical-align:-45%;line-height:0.mw-parser-output .quotebox-quote.quoted:afterfont-family:"Times New Roman",serif;font-weight:bold;font-size:large;color:gray;content:" ” ";line-height:0.mw-parser-output .quotebox .left-alignedtext-align:left.mw-parser-output .quotebox .right-alignedtext-align:right.mw-parser-output .quotebox .center-alignedtext-align:center.mw-parser-output .quotebox citedisplay:block;font-style:normal@media screen and (max-width:360px).mw-parser-output .quoteboxmin-width:100%;margin:0 0 0.8em!important;float:none!important
IUPAC definition
Note 1: The definition “crystal containing a second constituent which fits into and
is distributed in the lattice of the host crystal” given in refs.,[1][2] is not general
and, thus, is not recommended.
Note 2: The expression is to be used to describe a solid phase containing
more than one substance when, for convenience, one (or more) of the substances,
called the solvent, is treated differently from the other substances, called solutes.
Note 3: One or several of the components can be macromolecules. Some of
the other components can then act as plasticizers, i.e., as molecularly dispersed
substances that decrease the glass-transition temperature at which the amorphous
phase of a polymer is converted between glassy and rubbery states.
Note 4: In pharmaceutical preparations, the concept of solid solution is often
applied to the case of mixtures of drug and polymer.
Note 5: The number of drug molecules that do behave as solvent (plasticizer)
of polymers is small.[3]
A solid solution[4] is a solid-state solution of one or more solutes in a solvent. Such a multi-component system is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the chemical components remain in a single homogeneous phase. This often happens when the two elements (generally metals) involved are close together on the periodic table; conversely, a chemical compound generally results when two metals involved are not near each other on the periodic table.[5]
The solid solution needs to be distinguished from mechanical mixtures of powdered solids like two salts, sugar and salt, etc. The mechanical mixtures have total or partial miscibility gap in solid state.
Examples of solid solutions include crystallized salts from their liquid mixture, metal alloys, moist solids. In the case of metal alloys intermetallic compounds occur frequently.
Contents
1 Details
2 Application
3 Exsolution
4 See also
5 References
6 External links
Details
The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. Both of these types of solid solution affect the properties of the material by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material.[6]
Some mixtures will readily form solid solutions over a range of concentrations, while other mixtures will not form solid solutions at all. The propensity for any two substances to form a solid solution is a complicated matter involving the chemical, crystallographic, and quantum properties of the substances in question. Substitutional solid solutions, in accordance with the Hume-Rothery rules, may form if the solute and solvent have:
- Similar atomic radii (15% or less difference)
- Same crystal structure
- Similar electronegativities
- Similar valency a solid solution mixes with others to form a new solution
The phase diagram in Fig. 1 displays an alloy of two metals which forms a solid solution at all relative concentrations of the two species. In this case, the pure phase of each element is of the same crystal structure, and the similar properties of the two elements allow for unbiased substitution through the full range of relative concentrations.
Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.
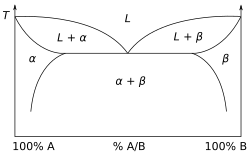
Fig. 2 This binary phase diagram shows two solid solutions: αdisplaystyle alpha
 and βdisplaystyle beta
and βdisplaystyle beta  .
.The binary phase diagram in Fig. 2 shows the phases of a mixture of two substances in varying concentrations, Adisplaystyle A















Application
In the phase diagram, at three different concentrations, the material will be solid until its heated to its melting point, and then (after adding the heat of fusion) become liquid at that same temperature:
- the unalloyed extreme left
- the unalloyed extreme right
- the dip in the center (the eutectic composition).
At other proportions, the material will enter a mushy or pasty phase until it warms up to being completely melted.
The mixture at the dip point of the diagram is called a eutectic alloy. Lead-tin mixtures formulated at that point (37/63 mixture) are useful when soldering electronic components, particularly if done manually, since the solid phase is quickly entered as the solder cools. In contrast, when lead-tin mixtures were used to solder seams in automobile bodies a pasty state enabled a shape to be formed with a wooden paddle or tool, so a 70-30 lead to tin ratio was used. (Lead is being removed from such applications owing to its toxicity and consequent difficulty in recycling devices and components that include lead.)
Exsolution
When a solid solution becomes unstable—due to a lower temperature, for example—exsolution occurs and the two phases separate into distinct microscopic to megascopic lamellae. This is mainly caused by difference in cation size. Cations which have a large difference in radii are not likely to readily substitute.[7]
Take the alkali feldspar minerals for example, whose end members are albite, NaAlSi3O8 and microcline, KAlSi3O8. At high temperatures Na+ and K+ readily substitute for each other and so the minerals will form a solid solution, yet at low temperatures albite can only substitute a small amount of K+ and the same applies for Na+ in the microcline. This leads to exsolution where they will separate into two separate phases. In the case of the alkali feldspar minerals, thin white albite layers will alternate between typically pink microcline,[7] resulting in a perthite texture.
See also
- Solid solution strengthening
- Vegard's law
References
^ Alan D. MacNaught; Andrew R. Wilkinson, eds. (1997). Compendium of Chemical Terminology: IUPAC Recommendations (2nd ed.). Blackwell Science. ISBN 0865426848..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Compendium of Analytical Nomenclature (the "Orange Book"). Oxford: Blackwell Science. 1998. ISBN 0865426155.
^ "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry. 84 (2): 377–410. 2012. doi:10.1351/PAC-REC-10-12-04.
^ "Solid solution - chemistry".
^ Cottrell, Alan Howard (1967). An Introduction to Metallurgy. Institute of Materials. ISBN 0-8448-0767-2.
^ Callister Jr., William D. (2006). Materials Science and Engineering: An Introduction (7th ed.). John Wiley & Sons. ISBN 0-471-35446-5.
^ ab Nesse, William D. (2000). Introduction to Mineralogy. New York: Oxford University Press. p91-92.
ISBN 978-0-19-510691-6
.mw-parser-output .refbeginfont-size:90%;margin-bottom:0.5em.mw-parser-output .refbegin-hanging-indents>ullist-style-type:none;margin-left:0.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>ddmargin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none.mw-parser-output .refbegin-100font-size:100%
Chen, Jing; Xu, Zhi-qin; Chen, Z-Z.; Li, T-F. & Chen, F-Y. (December 2005). "Pargasite and ilmenite exsolution texture in clinopyroxene from the Hujialing Garnet-Pyroxenite, Su-lu U.H.P. Terrane, Central China: A geodynamic Implication" (PDF). European Journal of Mineralogy. 17 (6): 895–903. Bibcode:2005EJMin..17..895C. doi:10.1127/0935-1221/2005/0017-0895. Archived from the original (PDF) on May 9, 2006.
Petersen, U. "Introduction to Ore Microscopy II; Mineral Paragenesis" (PDF). Archived from the original (PDF) on April 11, 2006.
External links
- DoITPoMS Teaching and Learning Package—"Solid Solutions"