Theaflavin

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP 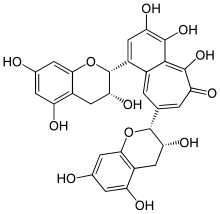 | |
| Names | |
|---|---|
IUPAC name 3,4,5-Trihydroxy-1,8-bis[(2R,3R)-3,5,7-trihydroxy-2-chromanyl]-6-benzo[7]annulenone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEMBL |
|
ChemSpider |
|
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C29H24O12 |
Molar mass | 564.50 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Theaflavin (TF) and its derivatives, known collectively as theaflavins, are antioxidant polyphenols that are formed from the condensation of flavan-3-ols in tea leaves during the enzymatic oxidation (sometimes erroneously referred to as fermentation) of black tea. Theaflavin-3-gallate, theaflavin-3'-gallate, and theaflavin-3-3'-digallate are the main theaflavins.[1] Theaflavins are types of thearubigins, and are therefore reddish in color. Epigallocatechin gallate (EGCG) will metabolize into some theaflavins in the liver.[citation needed] Those molecules contain a tropolone moiety.
Contents
1 Research into health effects
1.1 HIV
1.2 Cholesterol
1.3 Cancer
2 See also
3 References
Research into health effects
HIV
In in vitro laboratory investigations, several tea polyphenols, especially those with galloyl moiety, can inhibit HIV-1 replication with multiple mechanisms of action. Theaflavin derivatives have been found to have more potent anti-HIV-1 activity than catechin derivatives.[2]
Epigallocatechin gallate (EGCG), a catechin in green tea, binds to gp120, which works in conjunction with gp41, itself blocked by TF-3 (a theaflavin), both receptors of which HIV hijacks to enter into healthy human immune cells.[citation needed]
Cholesterol
In a human clinical trial published in 2003, theaflavins were found to reduce blood cholesterol levels, both total and LDL.[3]
Cancer
In in vitro laboratory investigations, theaflavins have been found to act on numerous points regulating cancer cell growth, survival, and metastasis.[4]
For example, TF-3 is a potent scavenger of superoxide.[5]
See also
- List of phytochemicals and foods in which they are prominent
- Theaflavin 3-gallate
References
^ "Theaflavin Effectiveness, Safety, and Drug Interactions on RxList". rxlist.com. Archived from the original on 4 September 2017. Retrieved 24 April 2018.
^ Liu S, Lu H, Zhao Q, et al. (2005). "Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41". Biochim. Biophys. Acta. 1723 (1–3): 270–81. doi:10.1016/j.bbagen.2005.02.012. PMID 15823507.
^ Maron DJ, Lu GP, Cai NS, et al. (2003). "Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial". Arch. Intern. Med. 163 (12): 1448–53. doi:10.1001/archinte.163.12.1448. PMID 12824094.
^ Bode AM, Dong Z (2006). "Molecular and Cellular Targets". Mol Carcinog. 45 (6): 422–430. doi:10.1002/mc.20222. PMC 2238808 . PMID 16688728.
. PMID 16688728.
^ Lin JK (2000). "Inhibition of Xanthine Oxidase and Suppression of Intracellular Reactive Oxygen Species in HL-60 Cells by Theaflavin-3,3'-digallate, (−)-Epigallocatechin-3-gallate, and Propyl Gallate". Journal of Agricultural and Food Chemistry. 48 (7): 2736–2743. doi:10.1021/jf000066d.