Gallocatechol

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP 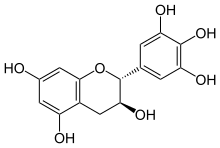 | |
| Names | |
|---|---|
| Other names (+)-gallocatechin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
MeSH | Gallocatechol |
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C15H14O7 |
Molar mass | 306.267 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position. It is one of the antioxidant chemicals found in food.
This compound possesses two epimers. The most common, (+)-gallocatechin (GC), CAS number 970-73-0, is found notably in green tea. Other sources of (+)-gallocatechin are bananas,[1]persimmons and pomegranates.[citation needed] The other enantiomer is called (-)-gallocatechin or ent-gallocatechin.
It was first isolated from green tea by Michiyo Tsujimura in 1934.[2]
This compound had been shown to have moderate affinity to the human cannabinoid receptor,[3] which may contribute to the health benefits found by consuming green tea.
Epigallocatechin is another type of catechin, with the gallate residue being in an isomeric cis position. It can be found in St John's wort.[4]
See also
- Epigallocatechin gallate
Ourateacatechin (4′-O-methyl-(−)-epigallocatechin)- Prodelphinidin
- List of phytochemicals in food
References
^ Antioxidant compounds from bananas (Musa cavendish). S. Someya, Y. Yoshiki and K. Okubo, Food Chemistry, Volume 79, Issue 3, November 2002, pp. 351-354, doi:10.1016/S0308-8146(02)00186-3
^ "Michiyo Tsujimura (1888–1969)". Ochanomizu University. Retrieved 10 November 2015.
^ Korte, G.; Dreiseitel, A.; Schreier, P.; Oehme, A.; Locher, S.; Geiger, S.; Heilmann, J.; Sand, P. (2010). "Tea catechins' affinity for human cannabinoid receptors". Phytomedicine. 17 (1): 19–22. doi:10.1016/j.phymed.2009.10.001. PMID 19897346.
^ Separation of epigallocatechin and flavonoids from Hypericum perforatum L. by high-speed counter-current chromatography and preparative high-performance liquid chromatography. Yun Wei, Qianqian Xie,Wanting Dong and Yoichiro Ito, Journal of Chromatography A, 1216 (2009), pages 4313–4318, doi:10.1016/j.chroma.2008.12.056
External links
- Epigallocatechin on the Sigma-Aldrich website
- Gallocatechin on the Sigma-Aldrich website
This article about a phenol is a stub. You can help Wikipedia by expanding it. |