Quercetin

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP 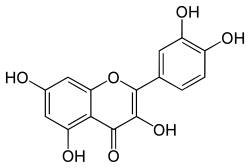 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈkwɜːrsɪtɪn/ |
IUPAC name 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | |
| Other names 5,7,3′,4′-flavon-3-ol, Sophoretin, Meletin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, Flavin meletin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.003.807 |
IUPHAR/BPS |
|
KEGG |
|
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C15H10O7 |
Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder[1] |
Density | 1.799 g/cm3 |
Melting point | 316 °C (601 °F; 589 K) |
Solubility in water | Practically insoluble in water; soluble in aqueous alkaline solutions[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |

UV visible spectrum of quercetin, with lambda max at 369 nm.
Quercetin, a plant flavonol from the flavonoid group of polyphenols, is found in many fruits, vegetables, leaves, and grains; red onions and kale are common foods containing appreciable content of quercetin.[2] It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
Contents
1 Occurrence
2 Biosynthesis
2.1 Glycosides
2.2 Rutin degradation pathway
3 Pharmacology
3.1 Pharmacokinetics
3.2 Metabolism
3.3 In vitro pharmacology
4 Health claims
4.1 Safety
5 See also
6 References
7 External links
Occurrence
Quercetin is a flavonoid widely distributed in nature.[2] The name has been used since 1857, and is derived from quercetum (oak forest), after Quercus.[3][4] It is a naturally occurring polar auxin transport inhibitor.[5]
Quercetin is one of the most abundant dietary flavonoids,[2][6] with an average daily consumption of 25–50 milligrams.[7]
| Foods | Quercetin (mg/100g) |
|---|---|
capers, raw | 234[6] |
capers, canned | 173[6] |
dock like sorrel | 86[6] |
radish leaves | 70[6] |
carob fiber | 58[6] |
| dill | 55[8] |
| cilantro | 53[6] |
| Hungarian wax pepper | 51[6] |
fennel leaves | 49[6] |
| onion, red | 32[6] |
| radicchio | 32[6] |
| watercress | 30[6] |
| kale | 23[6] |
| chokeberry | 19[6] |
| cranberry | 15[6] |
| lingonberry | 13[6] |
| plums, black | 12[6] |
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration.[9] One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit.[10] Quercetin is present in various kinds of honey from different plant sources.[11]
Biosynthesis
In plants, phenylalanine is converted to 4-coumaroyl-CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase.[12] One molecule of 4-coumaroyl-CoA is added to three molecules of malonyl-CoA to form tetrahydroxychalcone using 7,2′-dihydroxy-4′-methoxyisoflavanol synthase. Tetrahydroxychalcone is then converted into naringenin using chalcone isomerase.
Naringenin is converted into eriodictyol using flavanoid 3′-hydroxylase. Eriodictyol is then converted into dihydroquercetin with flavanone 3-hydroxylase, which is then converted into quercetin using flavonol synthase.[12]
Glycosides

3-O-Glycosides of quercetin
Quercetin is the aglycone form of a number of other flavonoid glycosides, such as rutin and quercitrin, found in citrus fruit, buckwheat and onions. Quercetin forms the glycosides quercitrin and rutin together with rhamnose and rutinose, respectively. Likewise guaijaverin is the 3-O-arabinoside, hyperoside is the 3-O-galactoside, isoquercitin is the 3-O-glucoside and spiraeoside is the 4′-O-glucoside. CTN-986 is a quercetin derivative found in cottonseeds and cottonseed oil. Miquelianin is the quercetin 3-O-β-D-glucuronopyranoside.[13]
Rutin degradation pathway
The enzyme quercitrinase can be found in Aspergillus flavus.[14] This enzyme hydrolyzes the glycoside quercitrin to release quercetin and L-rhamnose. It is an enzyme in the rutin catabolic pathway.[15]
Pharmacology
Pharmacokinetics
The bioavailability of quercetin in humans is low and highly variable (0–50%), and it is rapidly cleared with an elimination half-life of 1–2 hours after ingesting quercetin foods or supplements.[16] Following dietary ingestion, quercetin undergoes rapid and extensive metabolism that makes the biological effects presumed from in vitro studies unlikely to apply in vivo.[17][18]
Metabolism
In rats, quercetin did not undergo any significant phase I metabolism.[19] In contrast, quercetin did undergo extensive phase II (conjugation) to produce metabolites that are more polar than the parent substance and hence are more rapidly excreted from the body. The meta-hydroxyl group of catechol is methylated by catechol-O-methyltransferase. Four of the five hydroxyl groups of quercetin are glucuronidated by UDP-glucuronosyltransferase. The exception is the 5-hydroxyl group of the flavonoid ring which generally does not undergo glucuronidation. The major metabolites of orally absorbed quercetin are quercetin-3-glucuronide, 3'-methylquercetin-3-glucuronide, and quercetin-3'-sulfate.[19][20]
In vitro pharmacology
Quercetin has been reported to inhibit the oxidation of other molecules and hence is classified as an antioxidant.[17][20] Quercetin contains a polyphenolic chemical substructure that stops oxidation by acting as a scavenger of free radicals that are responsible for oxidative chain reactions.[21]
Quercetin also activates or inhibits the activities of a number of proteins.[22] For example, quercetin is a non-specific protein kinase enzyme inhibitor.[17][20] Quercetin has also been reported to have estrogenic (female sex hormone-like) activities by activating estrogen receptors. Quercetin activates both estrogen receptor alpha (ERα) and beta (ERβ)[23] with binding IC50 values of 1015 nM and 113 nM, respectively. Hence quercetin is somewhat ERβ selective (9 fold) and is roughly two to three orders of magnitude less potent than the endogenous estrogenic hormone 17β-estradiol.[24][25] In human breast cancer cell lines, quercetin has also been found to act as an agonist of the G protein-coupled estrogen receptor (GPER).[26][27]
Health claims
Quercetin has been studied in basic research and small clinical trials.[2][28][29] While quercetin supplements have been promoted for the treatment of cancer and various other diseases,[30] there is no evidence that quercetin (via supplements or in food) is useful to treat cancer[31] or any disease.[2][32] The US FDA has issued warning letters to emphasize that quercetin is neither a defined nutrient nor an antioxidant, cannot be assigned a dietary content level, and is not regulated as a drug to treat any human disease.[33][34]
Safety
In preliminary human studies, oral intake of quercetin in doses up to one gram per day over three months did not cause adverse effects.[2] The safety of using quercetin in dietary supplements during pregnancy and lactation has not been established.[2]
See also
|
|
|
References
^ abc Quercetin dihydrate safety sheet on http://www.pvp.com.br (English) Archived September 16, 2011, at the Wayback Machine.
^ abcdefg "Flavonoids (Review)". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. November 2015. Retrieved 1 April 2018.
^ "Quercetin". Merriam-Webster.
^ "Quercetin (biochemistry)". Encyclopædia Britannica.
^ Fischer C, Speth V, Fleig-Eberenz S, Neuhaus G (Oct 1997). "Induction of Zygotic Polyembryos in Wheat: Influence of Auxin Polar Transport". The Plant Cell. 9 (10): 1767–1780. doi:10.1105/tpc.9.10.1767. PMC 157020 . PMID 12237347.
. PMID 12237347.
^ abcdefghijklmnopq "USDA Database for the Flavonoid Content of Selected Foods, Release 3" (PDF). U.S. Department of Agriculture. 2011.
^ Formica JV, Regelson W (1995). "Review of the biology of quercetin and related bioflavonoids". Food and Chemical Toxicology. 33 (12): 1061–80. doi:10.1016/0278-6915(95)00077-1. PMID 8847003.
^ Justesen U, Knuthsen P (May 2001). "Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes". Food Chemistry. 73 (2): 245–50. doi:10.1016/S0308-8146(01)00114-5.
^ Slimestad R, Fossen T, Vågen IM (December 2007). "Onions: a source of unique dietary flavonoids". Journal of Agricultural and Food Chemistry. 55 (25): 10067–80. doi:10.1021/jf0712503. PMID 17997520.
^ Mitchell AE, Hong YJ, Koh E, Barrett DM, Bryant DE, Denison RF, Kaffka S (Jul 2007). "Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes". Journal of Agricultural and Food Chemistry. 55 (15): 6154–9. doi:10.1021/jf070344. PMID 17590007.
^ Petrus K, Schwartz H, Sontag G (Jun 2011). "Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry". Analytical and Bioanalytical Chemistry. 400 (8): 2555–63. doi:10.1007/s00216-010-4614-7. PMID 21229237.
^ ab Winkel-Shirley B (Jun 2001). "Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology". Plant Physiology. 126 (2): 485–93. doi:10.1104/pp.126.2.485. PMC 1540115 . PMID 11402179.
. PMID 11402179.
^ Juergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A (Nov 2003). "In vitro studies indicate that miquelianin (quercetin 3-O-beta-D-glucuronopyranoside) is able to reach the CNS from the small intestine". Planta Medica. 69 (11): 1013–7. doi:10.1055/s-2003-45148. PMID 14735439.
^ "Information on EC 3.2.1.66 - quercitrinase". BRENDA (BRaunschweig ENzyme DAtabase). Helmholtz Centre for Infection Research.
^ Tranchimand S, Brouant P, Iacazio G (Nov 2010). "The rutin catabolic pathway with special emphasis on quercetinase". Biodegradation. 21 (6): 833–59. doi:10.1007/s10532-010-9359-7. PMID 20419500.
^ Graefe EU, Derendorf H, Veit M (1999). "Pharmacokinetics and bioavailability of the flavonol quercetin in humans" (PDF). (review). International Journal of Clinical Pharmacology and Therapeutics. 37 (5): 219–33. PMID 10363620.
^ abc Williams RJ, Spencer JP, Rice-Evans C (Apr 2004). "Flavonoids: antioxidants or signalling molecules?". (review). Free Radical Biology & Medicine. 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
^ Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM (May 2011). "The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems". (review). Food & Function. 2 (5): 235–44. doi:10.1039/c1fo10025d. PMC 4122511 . PMID 21779561.
. PMID 21779561.
^ ab Day AJ, Rothwell JA, Morgan R (2004). "Characterization of polyphenol metabolites". In Bao Y, Fenwick R. Phytochemicals in health and disease. New York, NY: Dekker. pp. 50–67. ISBN 0-8247-4023-8.
^ abc Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, Palumbo R (2014). "Quercetin: a pleiotropic kinase inhibitor against cancer" (PDF). (review). Cancer Treatment and Research. 159: 185–205. doi:10.1007/978-3-642-38007-5_11. PMID 24114481.
^ Murakami A, Ashida H, Terao J (2008). "Multitargeted cancer prevention by quercetin". (review). Cancer Letters. 269 (2): 315–25. doi:10.1016/j.canlet.2008.03.046. PMID 18467024.
^ Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML, Nawroth R, Sanchez-Garcia I, Sharma D, Saxena NK, Singh N, Vlachostergios PJ, Guo S, Honoki K, Fujii H, Georgakilas AG, Bilsland A, Amedei A, Niccolai E, Amin A, Ashraf SS, Boosani CS, Guha G, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Azmi AS, Bhakta D, Halicka D, Keith WN, Nowsheen S (2015). "Sustained proliferation in cancer: Mechanisms and novel therapeutic targets". (review). Seminars in Cancer Biology. 35 Suppl: S25–54. doi:10.1016/j.semcancer.2015.02.006. PMID 25892662.
^ Moutsatsou P (2007). "The spectrum of phytoestrogens in nature: our knowledge is expanding". (review). Hormones (Athens, Greece). 6 (3): 173–93. PMID 17724002.
^ Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Andò S (2001). "Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells". (primary). Molecular Pharmacology. 60 (3): 595–602. PMID 11502892.
^ van der Woude H, Ter Veld MG, Jacobs N, van der Saag PT, Murk AJ, Rietjens IM (2005). "The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor". (primary). Molecular Nutrition & Food Research. 49 (8): 763–71. doi:10.1002/mnfr.200500036. PMID 15937998.
^ Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S (2004). "The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells". (primary). The Journal of Biological Chemistry. 279 (26): 27008–16. doi:10.1074/jbc.M403588200. PMID 15090535.
^ Prossnitz ER, Barton M (May 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". (review). Molecular and Cellular Endocrinology. 389 (1-2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308 . PMID 24530924.
. PMID 24530924.
^ Gross P (March 1, 2009), New Roles for Polyphenols. A 3-Part Report on Current Regulations & the State of Science, Nutraceuticals World
^ Miles SL, McFarland M, Niles RM (2014). "Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease". Nutrition Reviews. 72 (11): 720–34. doi:10.1111/nure.12152. PMID 25323953.
^ D'Andrea G (2015). "Quercetin: A flavonol with multifaceted therapeutic applications?". Fitoterapia. 106: 256–71. doi:10.1016/j.fitote.2015.09.018. PMID 26393898.
^ Ades TB, ed. (2009). Quercetin. American Cancer Society Complete Guide to Complementary and Alternative Cancer Therapies (2nd ed.). American Cancer Society. ISBN 9780944235713.
^ European Food Safety Agency (EFSA) NDA Panel (Dietetic Products, Nutrition and Allergies) (8 April 2011). "Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), "cardiovascular system" (ID 1844), "mental state and performance" (ID 1845), and "liver, kidneys" (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (4): 2067–82. doi:10.2903/j.efsa.2011.2067. Retrieved 24 September 2014.
^ Adams, AM (22 April 2014). "Warning Letter to River Hills Harvest dba Elderberrylife". Inspections, Compliance, Enforcement, and Criminal Investigations, US FDA. Retrieved 5 November 2014.
^ King, Janice A (2 March 2017). "Warning Letter to Cape Fear Naturals". Inspections, Compliance, Enforcement, and Criminal Investigations, US FDA. Retrieved 11 July 2017.
External links
 Media related to Quercetin at Wikimedia Commons
Media related to Quercetin at Wikimedia Commons