Stereoelectronic effect

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
In chemistry, primarily organic and computational chemistry, a stereoelectronic effect[1] is an effect on molecular geometry, reactivity, or physical properties due to spatial relationships in the molecules' electronic structure, in particular the interaction between atomic and/or molecular orbitals.[2] Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the ground and/or transition states of molecules that arise from considerations of orbital overlap.[3] Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientations of electrons (bonding or non-bonding) in space.[4][5]
Founded on a few general principles that govern how orbitals interact, the stereoelectronic effect, along with the steric effect, inductive effect, mesomeric effect, and aromaticity, constitutes an important class of rationalizations for observed patterns of selectivity, reactivity and stability in organic chemistry. In spite of the relatively straightforward premises, stereoelectronic effects often provide explanations for counterintuitive or surprising observations. As a result, stereoelectronic factors are now commonly considered and exploited in the development of new organic methodology and in the synthesis of complex targets. The scrutiny of stereoelectronic effects has also entered the realms of biochemistry and pharmaceutical chemistry in recent years.
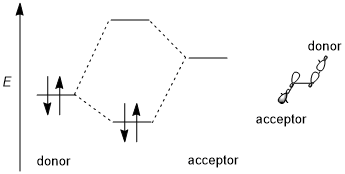
A stereoelectronic effect generally includes a stabilizing donor–acceptor (i.e., filled-empty, 2-electron 2-orbital) interaction. The donor is usually a higher bonding or nonbonding orbital and the acceptor is often a low-lying antibonding orbital as shown in the scheme below. Whenever possible, if this stereoelectronic effect is to be favored, the donor–acceptor orbitals should have (1) a small energy gap and (2) be geometrically well disposed for interaction. In particular, this means that the shapes of the donor and acceptor orbitals (including π or σ symmetry and size of the interacting lobes) must be well-matched for interaction; an antiperiplanar orientation is especially favorable. Occasionally, destabilizing donor-donor (i.e., filled-filled, 4-electron 2-orbital) interactions are also relevant factors.[3][6]
Important phenomena in which stereoelectronic effects may play an important role (or may even be dominant) include the anomeric effect and hyperconjugation.[6]
Contents
1 Trend of different orbitals
2 Influence on stability
3 Influence on conformation
3.1 Gauche effect
3.2 Special effects of fluorine substituent
4 Influence on reaction selectivity
4.1 Reductive cyclizations
4.2 Hydrogenation
5 Influence on thermodynamics
5.1 Influence on equilibrium
5.2 Influence on resonance structures
6 Application in asymmetric Diels–Alder reactions
7 Stereoelectronic effect versus steric clash
8 References
Trend of different orbitals
Take the simplest CH2X–CH3 system as an example; the donor orbital is σ(C–H) orbital and the acceptor is σ*(C–X). When moving from fluorine to chlorine, then to bromine, the electronegativity of the halogen and the energy level of the σ*(C–X) orbitals decreases.[7] Consequently, the general trend of acceptors can be summarized as: π*(C=O)>σ*(C–Hal)>σ*(C–O)>σ*(C–N)>σ*(C–C), σ*(C–H). For donating orbitals, the nonbonding orbitals, or the lone pairs, are generally more effective than bonding orbitals due to the high energy levels. Also, different from acceptors, donor orbitals require less polarized bonds. Thus, the general trends for donor orbitals would be: n(N)>n(O)>σ(C–C), σ(C–H)>σ(C–N)>σ(C–O)>σ(C–S)>σ(C–Hal).[6]

Stereoelectronic effect can be directional in specific cases. The radius of sulfur is much larger than the radius of carbon and oxygen. Thus the differences in C–S bond distances generate a much amplified difference in the two stereoelectronic effects in 1,3-dithiane (σ(C–H) → σ*(C–S)) than in 1,3-dioxane(σ(C–H) → σ*(C–O)).[7] The differences between C–C and C–S bonds shown below causes a significant difference in the distances between C–S and two C–H bonds. The shorter the difference is, the better the interaction and the stronger the stereoelectronic effect.[7]

Influence on stability
If there is an electropositive substituent (e.g. –SiR3, –SnR3, –HgR, etc.) at the β-position of carbocation, the positive charge could be stabilized which is also due largely to the stereoelectronic effect (illustrated below using –SiR3 as an example). The orientation of the two interacting orbitals can have a significant effect on the stabilization effect (σ(C–Si) → empty p orbital), where antiperiplanar (180°) > perpendicular (90°) > syn (0°).[8]
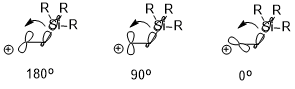
Influence on conformation
Gauche effect
One structural consequence of acyclic systems due to the stereoelectronic effect is the gauche effect.[9] In 1,2-difluoroethane, despite the steric clash, the preferred conformation is the gauche one because σ(C–H) is a good donor and σ*(C–F) is a good acceptor and the stereoelectronic effect (σ(C–H) → σ*(C–F)) requires the energy minimum to be gauche instead of anti.[10]

This gauche effect has a profound effect in bio-chemical research. In (2S,4R)-4-hydroxyproline fragment, the gauche interaction favors one the conformer that can bind selectively to the active site of pVHL, a domain in collagen, one of the most abundant animal protein structures and can lead to proteasomal degradation of HIF-α subunit.[11]

Special effects of fluorine substituent
The stereoelectronic effect can have a significant influence in pharmaceutical research. Generally, the substitution of hydrogen by fluorine could be regarded as a way to tune both the hydrophobicity and the metabolism rate. It has a profound influence on conformations. In anisole, the methyl group prefers the coplanar geometry with the phenyl group by about 3.0 kcal/mol, while the trifluoromethoxybenzene favors the O–CF3 bond to be perpendicular to the phenyl group. One explanation is the enhanced steric size of trifluoromethyl group compared to methyl group; yet, more importantly, once the trifluomethyl group rotates outside the phenyl plane, which orienting the C–F bond antiperiplanar (with the [C(aryl)–C(aryl)–O–C(F3)] dihedral angle in the energy minimized structure being around 90°) to the nonbonding orbital (the lone pair) on the oxygen atom which can benefit more of the stereoelectronic effect (n(O) → σ*(C–F)).[12]

Further studies illustrate that even for only one or two hydrogen atoms in a methyl group being replaced by a fluorine atom, the distortion in the structure can also be significant, with the [C(aryl)–C(aryl)–O–C(H2F)] dihedral angle in the energy minimized structure being around 24° and the [C(aryl)–C(aryl)–O–C(HF2)] dihedral angle 33°.[12]
Influence on reaction selectivity
Reductive cyclizations
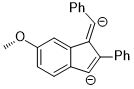
Although the energy difference between coplanar anisole and its isomer is quite large, the rotation between the O–CH3 bond becomes favorable when the electronic properties of methoxy group on aromatic rings need to be altered to stabilize an unusual intermediate or a transition state. In the following reaction, the regioselectivity could be rationalized as the out-of-plane rotation of the O–C bond which changes the methoxy group from an in-plane donor group to an out-of-plane acceptor group.[13]

The intermediate of the above reaction is the di-anion and the stereoelectronic effect that stabilizes this intermediate over the other one is the fact that the anionic charge at the para position could delocalize to the oxygen atom via orbital interaction: π(benzene) → σ*(O–CH3).[13]
Hydrogenation
Even remote substituents on the benzene ring can affect the electron density on the aromatic ring and in turn influence the selectivity. In the hydrogenation of ketones using CBS catalysts, the ketone coordinates to the boron atom with the lone pair on the oxygen atom. In the following example, the inductive influence of the substituents can lead to differentiation of the two sp2 lone pairs on the oxygen atom.[14]
The relevant stereoelectronic interaction in the starting material is the nO → σ*(Ccarbonyl–Caryl) interaction. The electron-withdrawing substituent on the benzene ring depletes the electron density on the aromatic ring and thus makes the σ*(Ccarbonyl–Caryl(nitro)) orbital a better acceptor than σ*(Ccarbonyl–Caryl(methoxy)). These two stereoelectronic interactions use different lone pairs on the oxygen atom (the one antiperiplanar to the σ* in question for each), leading to lone pairs with different electron densities. In particular, the enhanced depletion of electron density from the lone pair antiperiplanar to the 4-nitrophenyl group leads to weakened ability for that lone pair to coordinate to boron. This in turn results in the lone pair antiperiplanar to the 4-methoxyphenyl binding preferentially to the catalyst, leading to well-defined facial selectivity. Under optimized conditions, the product is formed with excellent levels of enantioselectivity (95% ee).[14]

Influence on thermodynamics
Influence on equilibrium
The stereoelectronic effect influences the thermodynamics of equilibrium and even between different resonance structures. For example, the following equilibrium could be achieved via a cascade of pericyclic reactions.
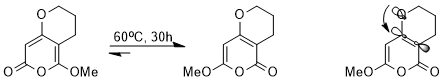
Though nearly every step is reversible, one of the two structures is strongly favored. With an oxygen atom in the ring, the double bonds cannot resonate with each other and are localized. As the σ*(C(sp2)–C(sp2)) orbital is a better acceptor than σ*(C(sp3)–C(sp3)), the stereoelectronic interaction of n(O) → σ*(C(sp2)–C(sp2)) has a much stronger stabilizing effect than n(O) → σ*(C(sp3)–C(sp3)); and thus the structure with better stereoelectronic interaction is favored thermodynamically in equilibrium.[15]
Another example of the preference in the equilibrium within the area of pericyclic reaction is shown below. The stereoelectronic effects that affect the quilibrium is the interaction between the delocalized “banana bonds” and the empty p orbital on the boron atom.[16]

Influence on resonance structures
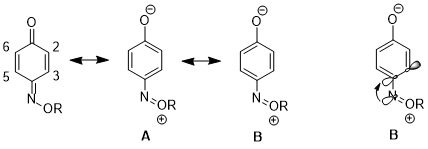
In another case, the stereoelectronic effect can result in a preference for one resonance structure over the other which leads to further consequences in reactivity. For 1,4-benzoquinone monoxime, significant differences separate the physical properties and reactivities between C2-C3 double bond and C5-C6 double bond with the J23 higher than J56.[17] The C2-C3 double bond is more isolated and tends not to undergo Diels–Alder reaction with ethylene.[18] This illustrates a preference of resonance structure B against structure A. The reason for the preference in 1,4-benzoquinone monoxime is the fact that the σ*(C(sp2)–C(sp2)) orbital is a better acceptor in single bonds than in double bonds. The stereoelectronic interaction of n(N) → σ*(C(sp2)–C(sp2)-single bond) is more favorable than n(N) → σ*(C(sp2)–C(sp2)-double bond).[19]
Application in asymmetric Diels–Alder reactions
In the asymmetric Diels–Alder reactions, instead of using chiral ligands or chiral auxiliaries to differentiate the side selectivity of the dienolphiles, the differentiation of face selectivity of the dienes (especially for cyclopentadiene derivatives) using stereoelectronic effects have been reported by Woodward since 1955.[20] A systematic research of facial selectivity using substituted cyclopentadiene or permethylcyclopentadiene derivatives have been conducted and the results can be listed as below.[21]

The stereoelectronic effect affecting the outcome of the facial selectivity of the diene in Diels–Alder reaction is the interaction between the σ(C(sp2)–CH3) (when σ(C(sp2)–X) is a better acceptor than a donor) or σ(C(sp2)–X) (when σ(C(sp2)–X) is a better donor than an acceptor) and the σ* orbital of the forming bond between the diene and the dienophile.[21]

If the two geminal substituents are both aromatic rings with different substituents tuning the electron density, the differentiation of the facial selectivity is also facile where the dienophile approaches to the diene anti to the more electron-rich C–C bond where the stereoelectronic effect in this case is similar to the previous one.[22]
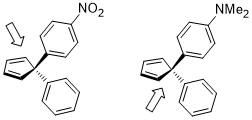
The ring opening of cyclobutene under heating conditions can have two products: inward and outward rotation.

The inward rotation transition state of the second shown below is relatively favored for acceptor R substituents (e.g. NO2) but is especially disfavored by donor R substituents (e.g. NMe2).[23]

Stereoelectronic effect versus steric clash
Sometimes, stereoelectronic effects can win over extreme steric clash. In a similar cyclobutene ring-opening reaction, the trimethylsilyl group, which is very bulky, still favors the inward rotation. The stereoelectronic effect, which is the interaction shown above when the acceptor orbital is the σ*(Si–CH3), appears to be a more predominant factor in determining the reaction selectivity against the steric hindrance and even wins over the penalty of the disrupted conjugation system of the product due to steric clash.[24]
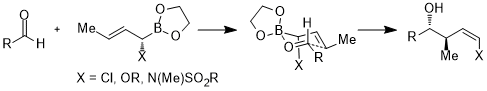
Furthermore, the acceptor orbitals are not limited to the antibonding orbitals of carbon-heteroatom bonds or the empty orbitals; in the following case, the acceptor orbital is the σ*(B–O) orbital. In the six-membered ring transition state, the stereoelectronic interaction is σ(C–X) → σ*(B–O).[25]
References
^ Alabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1118906349.html
^ Cramer, Christopher J. (1996). "Hyperconjugation as it affects conformational analysis". Journal of Molecular Structure: THEOCHEM. 370 (2–3): 135–146. doi:10.1016/S0166-1280(96)04567-8. ISSN 0166-1280..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ ab Evans, D. A. (2006). Chemistry 206 Lecture Notes. Cambridge, MA: Harvard University (Unpublished). pp. 1–2 (Lecture 1).
^ Pierre., Deslongchamps, (1983). Stereoelectronic effects in organic chemistry (1st ed.). Oxford [Oxfordshire]: Pergamon Press. ISBN 0080261841. OCLC 9412829.
^ A fully rigorous analysis of stereoelectronic effects would involve the analysis of interactions between symmetry-correct canonical molecular orbitals. However, in practice, many chemists (e.g., Evans, Fleming, Kirby) use a localized molecular orbital model to analyze stereoelectronic effects. Deslongchamps goes so far as to describe stereoelectronic effects as originating from the stereochemical relationship of electron pairs. It should be noted that orbital interactions invoked in a stereoelectronic effect can generally be 'translated' to an equally valid classical valence bond/resonance form picture, though usually involving 'no-bond' resonance forms.
^ abc Kirby, A. J. (1996). Stereoelectronic Effects. Oxford: Oxford University Press. ISBN 9780198558934.
^ abc Alabugin, Igor V.; Zeidan, Tarek A. (2002). "Stereoelectronic Effects and General Trends in Hyperconjugative Acceptor Ability of σ Bonds". Journal of the American Chemical Society. 124 (12): 3175–3185. doi:10.1021/ja012633z. ISSN 0002-7863. PMID 11902907.
^ Carey, F. A.; Sundberg, R. J. (2007). Advanced Organic Chemistry, Part A: Structure and Mechanism (5th ed.). New York: Springer. ISBN 0387448977.
^ Hanack, M. (1965). Conformational Theory. New York and London: Academic Press.
^ Anslyn, E. V.; Dougherty, D. A. (2004). Modern Physical Organic Chemistry. Sausalito, Calif.: University Science Books. ISBN 1891389319.
^ Fujimori, D.G. (2009). "Hypoxia sensing goes gauche". Nat. Chem. Biol. 5 (4): 202–203. doi:10.1038/nchembio0409-202.
^ ab Müller, K.; Faeh, C.; Diederich, F. (2007). "Fluorine in Pharmaceuticals: Looking Beyond Intuition". Science. 317 (5846): 1881–1886. Bibcode:2007Sci...317.1881M. doi:10.1126/science.1131943. ISSN 0036-8075. PMID 17901324.
^ ab Peterson, Paul W.; Shevchenko, Nikolay; Alabugin, Igor V. (2013). ""Stereoelectronic Umpolung": Converting a p-Donor into a σ-Acceptor via Electron Injection and a Conformational Change". Organic Letters. 15 (9): 2238–2241. doi:10.1021/ol400813d. ISSN 1523-7060. PMID 23639080.
^ ab Corey, E.J.; Helal, Christopher J. (1995). "Novel electronic effects of remote substituents on the oxazaborolidine-catalyzed enantioselective reduction of ketones". Tetrahedron Letters. 36 (50): 9153–9156. doi:10.1016/0040-4039(95)01961-G. ISSN 0040-4039.
^ Venkataraman, Hemalatha; Cha, Jin K. (1989). "The scope and mechanism of rearrangement of 4,6-dialkoxy-2-pyrones". Tetrahedron Letters. 30 (27): 3509–3512. doi:10.1016/S0040-4039(00)99426-7. ISSN 0040-4039.
^ Gridnev, Ilya D.; Tok, Oleg L.; Gridneva, Natalya A.; Bubnov, Yuri N.; Schreiner, Peter R. (1998). "Synthesis and Dynamic Properties of Cycloheptatrienyl(dipropyl)borane. Equilibrium with 7-Dipropylborylnorcaradiene". Journal of the American Chemical Society. 120 (5): 1034–1043. doi:10.1021/ja9724699. ISSN 0002-7863.
^ Norris, R. K.; Sternhell, S. (1969). "2-Substituted and 2,6-disubstituted 1,4-benzoquinone 4-oximes ("p-nitrosophenols")". Aust. J. Chem. 22 (5): 935–970. doi:10.1071/CH9690935.
^ Baldwin, J. E.; Norris, R. K. (1981). "Stereoelectronic control in organic chemistry: addition reactions of some 1,4-benzoquinone 4-(O-methyloximes)". J. Org. Chem. 46: 697–703. doi:10.1021/jo00317a011.
^ Perrin, C. L.; Engler, R. E. (1997). "Origin of Apparent Stereoelectronic Effects in Structure and Reactivity of Benzoquinone Monooximes". J. Org. Chem. 62: 687–692. doi:10.1021/jo961386s.
^ Winstein, S.; Shatavsky, M.; Norton, C.; Woodward, R. B. (1955). "7-NORBORNENYL AND 7-NORBORNYL CATIONS". J. Am. Chem. Soc. 77: 4183–4184. doi:10.1021/ja01620a078.
^ ab Mehta, Goverdhan; Uma, R. (2000). "Stereoelectronic Control in Diels−Alder Reaction of Dissymmetric 1,3-Dienes". Accounts of Chemical Research. 33 (5): 278–286. doi:10.1021/ar990123s. ISSN 0001-4842. PMID 10813872.
^ Halterman, R. L.; McCarthy, B. A.; McEvoy, M. A. (1992). "Stereoelectronic control of facial selectivity in the Diels-Alder cycloaddition of sterically unbiased 5,5-diarylcyclopentadienes". J. Org. Chem. 57: 5585–5589. doi:10.1021/jo00047a009.
^ Shindo, M.; Sato, Y.; Shishido, J. (1998). "A highly stereoselective synthesis of tri- and tetrasubstituted olefins via ynolates". Tetrahedron Lett. 39: 4857–4860. doi:10.1016/s0040-4039(98)00921-6.
^ Murakami, M.; Hasegawa, M. (2004). "Synthesis and Thermal Ring Opening oftrans-3,4-Disilylcyclobutene". Angew. Chem. Int. Ed. 43: 4874–4876. doi:10.1002/anie.200460144.
^ Schlapbach, A.; Hoffmann, R. W. (2001). "(E)-α-Sulfonamidocrotylboronates as Reagents for the Stereoselective Homoaldol Synthesis". J. Org. Chem. 66: 323–328. doi:10.1002/1099-0690(200101)2001:2<323::aid-ejoc323>3.0.co;2-a.