Racemic mixture

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
In chemistry, a racemic mixture, or racemate (/reɪˈsiːmeɪt, rə-, ˈræsɪmeɪt/)[1], is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. A sample with only a single enantiomer is an enantiomerically pure or enantiopure compound.[2]
Contents
1 Etymology
2 Nomenclature
3 Properties
4 Crystallization
5 Resolution
6 Synthesis
7 Racemic pharmaceuticals
8 Wallach's rule
9 See also
10 References
Etymology
From racemic acid found in grapes; from Latin racemus, meaning a bunch of grapes.
Nomenclature
A racemic mixture is denoted by the prefix (±)- or dl- (for sugars the prefix .mw-parser-output .smallcapsfont-variant:small-caps
dl- may be used), indicating an equal (1:1) mixture of dextro and levo isomers. Also the prefix rac- (or racem-) or the symbols RS and SR (all in italic letters) are used.
If the ratio is not 1:1 (or is not known), the prefix (+)/(−),
d/l- or d/l- (with a slash) is used instead.
The usage of d and l is strongly discouraged by IUPAC.[3][4]
Properties
A racemate is optically inactive, meaning that there is no net rotation of plane-polarized light. Although the two enantiomers rotate plane-polarized light in opposite directions, the rotations cancel because they are present in equal amounts.
In contrast to the two pure enantiomers, which have identical physical properties except for the direction of rotation of plane-polarized light, a racemate sometimes has different properties from either of the pure enantiomers. Different melting points are most common, but different solubilities and boiling points are also possible.
Pharmaceuticals may be available as a racemate or as the pure enantiomer, which might have different potencies. Because biological systems have many chiral asymmetries, pure enantiomers frequently have very different biological effects; examples include glucose and methamphetamine.
Crystallization
There are four ways in which a racemate can be crystallized, depending on the substance; three of which H. W. B. Roozeboom had distinguished by the year 1899:
- Conglomerate (sometimes racemic conglomerate)
- If the 'molecules' of the substance have a much greater affinity for the same enantiomer than for the opposite one, a mechanical mixture of enantiomerically pure crystals will result. The melting point of the racemic conglomerate is always lower than that of the pure enantiomer. Addition of a small amount of one enantiomer to the conglomerate increases the melting point. Roughly 10% of racemic chiral compounds crystallize as conglomerates.
- Racemic compound (sometimes true racemate)
- If molecules have a greater affinity for the opposite enantiomer than for the same enantiomer, the substance forms a single crystalline phase in which the two enantiomers are present in an ordered 1:1 ratio in the elementary cell. Adding a small amount of one enantiomer to the racemic compound decreases the melting point. But the pure enantiomer can have a higher or lower melting point than the compound. A special case of racemic compounds are kryptoracemates, in which the crystal itself has handedness (is enantiomorphic), despite containing both enantiomorphs in a 1:1 ratio.[5]
- Pseudoracemate (sometimes racemic solid solution)
- When there is no big difference in affinity between the same and opposite enantiomers, then in contrast to the racemic compound and the conglomerate, the two enantiomers will coexist in an unordered manner in the crystal lattice. Addition of a small amount of one enantiomer changes the melting point just little bit or not at all.
- Quasiracemate
- A quasiracemate is a co-crystal of two similar but distinct compounds, one of which is left-handed and the other right-handed. Although chemically different, they are sterically similar (isosteric) and are still able to form a racemic crystalline phase. One of the first such racemates studied, by Pasteur in 1853, forms from a 1:2 mixture of the bis ammonium salt of (+)-tartaric acid and the bis ammonium salt of (−)-malic acid in water. Re-investigated in 2008,[6] the crystals formed are dumbbell-shape with the central part consisting of ammonium (+)-bitartrate, whereas the outer parts are a quasiracemic mixture of ammonium (+)-bitartrate and ammonium (−)-bimalate.
Resolution
The separation of a racemate into its components, the pure enantiomers, is called a chiral resolution. There are various methods, including crystallization, chromatography, and the use of enzymes. The first successful resolution of a racemate was performed by Louis Pasteur, who manually separated the crystals of a conglomerate.
Synthesis
Without a chiral influence (for example a chiral catalyst, solvent or starting material), a chemical reaction that makes a chiral product will always yield a racemate. That can make the synthesis of a racemate cheaper and easier than making the pure enantiomer, because it does not require special conditions. This fact also leads to the question of how biological homochirality evolved on what is presumed to be a racemic primordial earth.
The reagents of, and the reactions that produce, racemic mixtures are said to be "not stereospecific" or "not stereoselective", for their indecision in a particular stereoisomerism. A frequent scenario is that of a planar species (such as an sp2 carbon atom or a carbocation intermediate) acting as an electrophile. The nucleophile will have a 50% probability of 'hitting' either of the two sides of the planar grouping, thus producing a racemic mixture:
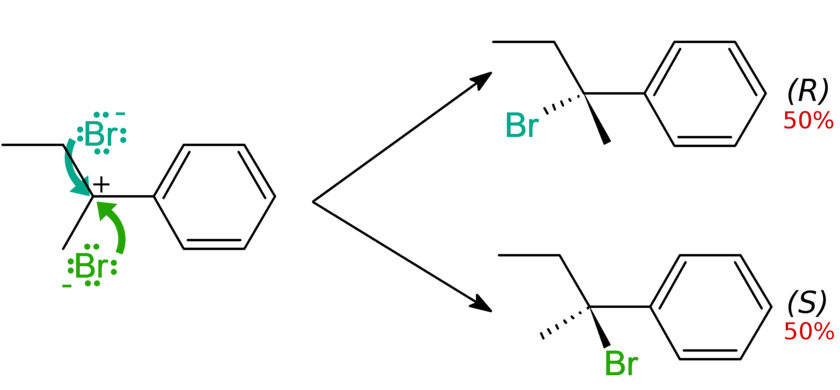
Racemic pharmaceuticals
Some drug molecules are chiral, and the enantiomers have different effects on biological entities. They can be sold as one enantiomer or as a racemic mixture. Examples include thalidomide, ibuprofen, and salbutamol. Adderall is an unequal mixture of both amphetamine enantiomers. A single amphetamine dose combines the neutral sulfate salts of dextroamphetamine and amphetamine, with the dextro isomer of amphetamine saccharate and D/L-amphetamine aspartate monohydrate. The prescription analgesic tramadol is also a racemate.
In some cases (e.g., ibuprofen and thalidomide), the enantiomers interconvert or racemize in vivo. This means that preparing a pure enantiomer for medication is largely pointless. However, sometimes samples containing pure enantiomers may be made and sold at a higher cost in cases where the use requires specifically one isomer (e.g., for a stereospecific reagent); compare omeprazole and esomeprazole.
While often only one enantiomer of the drug may be active, in cases like salbutamol[7] and thalidomide, the other enantiomer may be harmful. The (R) enantiomer of thalidomide is effective against morning sickness, while the (S) enantiomer is teratogenic, causing birth defects. Since the drug racemizes, the drug cannot be considered safe for use by women of child-bearing age,[8] and its use is tightly controlled when used for treating other illness.[9]
Methamphetamine is available by prescription under the brand name Desoxyn. The active component of Desoxyn is dextromethamphetamine hydrochloride. This is the right-handed isomer of methamphetamine. The left-handed isomer of methamphetamine, levomethamphetamine, is an OTC drug that is less centrally-acting and more peripherally-acting.
Wallach's rule
Wallach's rule (first proposed by Otto Wallach) states that racemic crystals tend to be more dense than their chiral counterparts.[10] This rule has been substantiated by crystallographic database analysis.[11]
See also
Chirality (same as optical isomerism)- Racemization
- Racemic (protein) crystallography
References
^ "Racemate". Merriam-Webster Dictionary. Retrieved 8 July 2018..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Moss, Gerry P. (1996). Basic terminology of stereochemistry (IUPAC Recommendations 1996). Department of Chemistry, Queen Mary University of London: Blackwell Scientific Publications. pp. 8, 11.
^ G.P. Moss: Basic terminology of stereochemistry ( Recommendations 1996); Pure Appl. Chem., 1996, Vol. 68, No. 12, p. 2205-2216; doi:10.1351/pac199668122193
^ Nomenclature of Carbohydrates (Recommendations 1996), 2-Carb-4. – Configurational symbols and prefixes
^ L. Fábián & C. P. Brock (2010). "A list of organic kryptoracemates". Acta Crystallogr. B66 (1): 94–103. doi:10.1107/S0108768109053610. PMID 20101089.
^ Rediscovering Pasteur's Quasiracemates Kraig A. Wheeler, Rebecca C. Grove, Raymond E. Davis, and W. Scott Kassel Angew. Chem. Int. Ed. 2008, 47, 78 –81 doi:10.1002/anie.200704007
^ (R)-Albuterol for Asthma: Pro, Bill T. Ameredes and William J. Calhoun
^ "Use of thalidomide in leprosy". WHO:leprosy elimination. WHO. Retrieved 22 April 2010.
^ Sheryl Gay Stolberg (17 July 1998). "Thalidomide Approved to Treat Leprosy, With Other Uses Seen". The New York Times. Retrieved 8 January 2012.
^ (Wallach, O. (1895). Liebigs Ann. Chem. 286, 90–143.)
^ Carolyn Pratt Brock, W. Bernd Schweizer, and Jack D. Dunitz (1991). "On the validity of Wallach's rule: on the density and stability of racemic crystals compared with their chiral counterparts". J. Am. Chem. Soc. 113 (26): 9811–9820. doi:10.1021/ja00026a015.CS1 maint: Multiple names: authors list (link)
