Diazonium compound

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
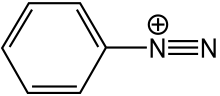
Benzenediazonium cation
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N+
2X−
where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen. Alkyldiazonium compounds are generally unstable and nonisolable, due to the extreme leaving group ability of N2 in SN1/E1 (secondary and tertiary alkyldiazonium salts) or SN2 (methyl and primary alkyldiazonium salts) substitution and elimination reactions. They have been used as substrates in physical organic chemistry studies, but their uncontrolled reactivity generally renders them synthetically unimportant. (As an exception, a methyldiazonium carboxylate ion pair is believed to be a fleeting intermediate in the methylation of carboxylic acids by diazomethane). On the other hand, aryldiazonium salts are more stable (though still dangerously explosive under certain conditions) and are highly versatile reagents for chemical synthesis and important intermediates in the organic synthesis of azo dyes.[1]
Contents
1 Preparation
2 Diazo coupling reactions
3 Displacement of the N2 group
3.1 Replacement by Halogens
3.1.1 Sandmeyer reaction
3.1.2 Gatterman reaction
3.1.3 Replacement by iodide
3.1.4 Replacement by fluoride
3.2 Miscellaneous Replacements
3.2.1 Replacement by hydrogen
3.2.2 Replacement by a hydroxyl group
3.2.3 Replacement by a nitro group
3.2.4 Replacement by a cyano group
3.2.5 Replacement by a trifluoromethyl group
3.2.6 Replacement by a thiol group
3.2.7 Replacement by an aryl group
3.2.8 Replacement by boronate ester group
4 Meerwein reaction
5 Metal complexes
6 Other methods for dediazotization
7 Grafting reactions
8 Reduction to a hydrazine group
9 Applications
9.1 Other uses
10 Safety
11 See also
12 References
13 External links
Preparation
The process of forming diazonium compounds is called "diazotation", "diazoniation", or "diazotization". The reaction was first reported by Peter Griess in 1858, who subsequently discovered several reactions of this new class of compounds. Most commonly, diazonium salts are prepared by treatment of aromatic amines with nitrous acid and additional acid. Usually the nitrous acid is generated in situ (in the same flask) from sodium nitrite and the excess mineral acid (usually aqueous HCl, H2SO4, p-H3CC6H4SO3H, or HBF4):
- ArNH2 + HNO2 + HX → ArN2+X– + 2H2O

Sample of phenyldiazonium tetrafluoroborate.
Aqueous solutions of diazonium chloride salts, traditionally prepared from the aniline, sodium nitrite, and hydrochloric acid, are unstable at room temperature and are classically prepared at 0 – 5 °C. However, one can isolate diazonium compounds as tetrafluoroborate or tosylate salts,[2] which are stable solids at room temperature. It is often preferred that the diazonium salt remain in solution, but they do tend to supersaturate. Operators have been injured or even killed by an unexpected crystallization of the salt followed by its detonation.[3]
Due to these hazards, diazonium compounds are usually not isolated. Instead they are used in situ. This approach is illustrated in the preparation of an arylsulfonyl compound:[4]
Diazo coupling reactions
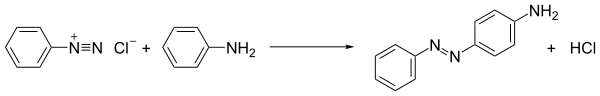
The most widely practiced reaction of diazonium salts is azo coupling. In this process, the diazonium compound is attacked by, i.e., coupled to, electron-rich substrates. When the coupling partners are arenes such as anilines and phenols, the process is an example of electrophilic aromatic substitution:
ArN+
2 + Ar′H → ArN2Ar′ + H+
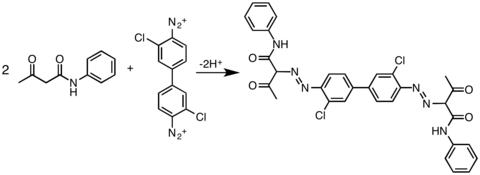
Another commercially important class of coupling partners are acetoacetic amides, as illustrated by the preparation of Pigment Yellow 12, a diarylide pigment.[5]
The resulting azo compounds are often useful dyes and in fact are called azo dyes.[6] The deep colors of the dyes reflects their extended conjugation. For example, the dye called aniline yellow is produced by mixing aniline and cold solution of diazonium salt and then shaking it vigorously. Aniline yellow is obtained as an yellow solid.[7] Similarly, a cold basic solution of Naphthalen-2-ol (Β-naphthol) give the intensely orange-red precipitate.[7]Methyl orange is an example of an azo dye that is used in the laboratory as a pH indicator.
Displacement of the N2 group
Arenediazonium cations undergo several reactions in which the N2 group is replaced by another group or ion. Some of the major ones are the following.[8][9]
Replacement by Halogens
Sandmeyer reaction
Benzenediazonium chloride heated with cuprous chloride or cuprous bromide respectively dissolved in HCl or HBr yield chlorobenzene or bromobenzene, respectively.
C
6H
5N+
2 + CuCl → C6H5Cl + N2 + Cu+
Gatterman reaction
In the Gatterman reaction, benzenediazonium chloride is warmed with copper powder and HCl or HBr to produce chlorobenzene and bromobenzene respectively. It is named after the German chemist Ludwig Gattermann.[10]
- 2Cu + 2C6H5N2+ → 2Cu+ + H5C6–C6H5 + 2N2 (initiation)
C
6H
5N+
2 + HX → C6H5X + N2 + H+ (Cu+ catalysis)
Replacement by iodide
Iodine is not easily introduced into the benzene ring directly. However it can be introduced by treating aryldiazonium cations with potassium iodide:
C
6H
5N+
2 + KI → C6H5I + K+ + N2
Replacement by fluoride
Fluorobenzene is produced by thermal decomposition of benzenediazonium fluoroborate. The conversion is called the Balz-Schiemann reaction.[11]
- [C
6H
5N+
2]BF−
4 → C6H5F + BF3 + N2
Miscellaneous Replacements
Replacement by hydrogen
Arenediazonium cations are reduced by hypophosphorous acid or sodium stannite gives benzene:
- [C
6H
5N+
2]Cl− + H3PO2 + H2O → C6H6 + N2 + H3PO3 + HCl
Replacement by a hydroxyl group
Phenols are produced by heating aqueous solutions of aryldiazonium salts:[12][13]
C
6H
5N+
2 + H2O → C6H5OH + N2 + H+
This conversion is often an unwanted side reaction.[14] This reaction goes by the German name Phenolverkochung ("cooking down to yield phenols"). The phenol formed may react with the diazonium salt and hence the reaction is carried in the presence of an acid which helps in suppressing this further reaction.
The reaction has been applied to the preparation dihydroxyl analogues of the Tröger’s base.[12] A Sandmeyer-type hydroxylation is also possible using Cu2O and Cu2+ in water.
Replacement by a nitro group
Nitrobenzene can be obtained by treating benzenediazonium fluoroborate with sodium nitrite in presence of copper. Alternatively, the diazotisation of the aniline can be conducted in presence of cuprous oxide, which generates cuprous nitrite in situ:
C
6H
5N+
2 + CuNO2 → C6H5NO2 + N2 + Cu+
Replacement by a cyano group
The cyano group usually cannot be introduced by nucleophilic substitution of haloarenes, but such compounds can be easily prepared from diazonium salts. Illustrative is the preparation of benzonitrile using the reagent cuprous cyanide:
C
6H
5N+
2 + CuCN → C6H5CN + Cu+ + N2
This reaction is a special type of Sandmeyer reaction.
Replacement by a trifluoromethyl group
Two research groups reported trifluoromethylations of diazonium salts in 2013. Goossen reported the preparation of a CuCF3 complex from CuSCN, TMSCF3, and Cs2CO3. In contrast, Fu reported the trifluoromethylation using Umemoto's reagent (S-trifluoromethyldibenzothiophenium tetrafluoroborate) and Cu powder (Gattermann-type conditions). They can be described by the following equation:
- C6H5N2+ + [CuCF3] → C6H5CF3 + [Cu]+ + N2
The bracket indicates that other ligands on copper are likely present but are omitted.
Replacement by a thiol group
Diazonium salts can be converted to thiols in a two-step procedure. Treatment of benzenediazonium chloride with potassium ethylxanthate followed by hydrolysis of the intermediate xanthate ester gives thiophenol:
C
6H
5N+
2 + C
2H
5OCS−
2 → C6H5SC(S)OC2H5- C6H5SC(S)OC2H5 + H2O → C6H5SH + HOC(S)OC2H5
Replacement by an aryl group
The aryl group can be coupled to another using aryldiazonium salts. For example, treatment of benzenediazonium chloride with benzene (an aromatic compound) in the presence of sodium hydroxide gives diphenyl:
- [C
6H
5N+
2]Cl− + C6H6 → C6H5−C6H5 + N2 + HCl
This reaction is known as the Gomberg–Bachmann reaction. A similar conversion is also achieved by treating benzenediazonium chloride with ethanol and copper powder.
Replacement by boronate ester group
A Bpin (pinacolatoboron) group, of use in Suzuki-Miyaura cross coupling reactions, can be installed by reaction of a diazonium salt with bis(pinacolato)diboron in the presence of benzoyl peroxide (2 mol %) as an initator:[15]
- C6H5N2+X– + pinB–Bpin → C6H5Bpin + X–Bpin + N2
Meerwein reaction
Benzenediazonium chloride reacts with compounds containing activated double bonds to produces phenylated products. The reaction is called the Meerwein arylation:
- [C
6H
5N+
2]Cl− + ArCH=CHCO2H → ArC=C−C6H5 + N2 + CO2 + HCl
Metal complexes
In their reactions with metal complexes, diazonium cations behave similarly to NO+. For example, low-valent metal complexes add with diazonium salts. Illustrative complexes are [Fe(CO)2(PPh3)2(N2Ph)]+ and the chiral-at-metal complex Fe(CO)(NO)(PPh3)(N2Ph).[16]
Other methods for dediazotization
- by organic reduction at an electrode
- by mild reducing agents such as ascorbic acid (vitamin C)[17]
- by gamma radiation from solvated electrons generated in water
photoinduced electron transfer- reduction by metal cations, most commonly a cuprous salt.
- anion-induced dediazoniation: a counterion such as iodine gives electron transfer to the diazonium cation forming the aryl radical and an iodine radical
- solvent-induced dediazoniation with solvent serving as electron donor
Grafting reactions
In a potential application in nanotechnology, the diazonium salts 4-chlorobenzenediazonium tetrafluoroborate very efficiently functionalizes single wall nanotubes.[18] In order to exfoliate the nanotubes, they are mixed with an ionic liquid in a mortar and pestle. The diazonium salt is added together with potassium carbonate, and after grinding the mixture at room temperature the surface of the nanotubes are covered with chlorophenyl groups with an efficiency of 1 in 44 carbon atoms. These added subsituents prevent the tubes from forming intimate bundles due to large cohesive forces between them, which is a recurring problem in nanotube technology.
It is also possible to functionalize silicon wafers with diazonium salts forming an aryl monolayer. In one study, the silicon surface is washed with ammonium hydrogen fluoride leaving it covered with silicon–hydrogen bonds (hydride passivation).[19] The reaction of the surface with a solution of diazonium salt in acetonitrile for 2 hours in the dark is a spontaneous process through a free radical mechanism:[20]
So far grafting of diazonium salts on metals has been accomplished on iron, cobalt, nickel, platinum, palladium, zinc, copper and gold surfaces. Also grafting to diamond surfaces has been reported.[21] One interesting question raised is the actual positioning on the aryl group on the surface. An in silico study [22] demonstrates that in the period 4 elements from titanium to copper the binding energy decreases from left to right because the number of d-electrons increases. The metals to the left of iron are positioned tilted towards or flat on the surface favoring metal to carbon pi bond formation and those on the right of iron are positioned in an upright position, favoring metal to carbon sigma bond formation. This also explains why diazonium salt grafting thus far has been possible with those metals to right of iron in the periodic table.
Reduction to a hydrazine group
Diazonium salts can be reduced with stannous chloride (SnCl2) to the corresponding hydrazine derivatives. This reaction is particularly useful in the Fischer indole synthesis of triptan compounds and indometacin. The use of sodium dithionite is an improvement over stannous chloride since it is a cheaper reducing agent with fewer environmental problems.
Applications
The first use of diazonium salts was to produce water-fast dyed fabrics by immersing the fabric in an aqueous solution of the diazonium compound, followed by immersion in a solution of the coupler (the electron-rich ring that undergoes electrophilic substitution). The major applications of diazonium compounds remains in the dye and pigment industry.[6]
Other uses
Diazonium compounds are standard reagents used in synthesis of organic compounds, especially aryl derivatives.
Diazonium salts are light sensitive and break down under near UV or violet light. This property has led to their use in document reproduction. In this process, paper or film is coated with a diazonium salt. After contact exposure under light, the residual diazo is converted to a stable azo dye with an aqueous solution of coupler. A more common process uses a paper coated with diazo, coupler and an acid to inhibit coupling; after exposure the image is developed by a vapor mixture of ammonia and water which forces coupling.
Safety
Solid diazonium halides are often dangerously explosive, and fatalities and injuries have been reported.[3]
The nature of the anions affects stability of the salt. Aryl diazonium perchlorates, such as nitrobenzenediazonium perchlorate, have been used to initiate explosives.
See also
- Diazo
- Benzenediazonium chloride
- Triazene cleavage
- Dinitrogen complex
References
^ Chemistry of the Diazonium and Diazo Groups: Part 1. S. Patai, Ed. 1978 Wiley-Blackwell. .mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
ISBN 0-471-99492-8. Chemistry of the Diazonium and Diazo Groups: Part 2. S. Patai, Ed. 1978 Wiley-Blackwell.
ISBN 0-471-99493-6.
^ Filimonov, Victor D.; Trusova, Marina; Postnikov, Pavel; Krasnokutskaya, Elena A.; Lee, Young Min; Hwang, Ho Yun; Kim, Hyunuk; Chi, Ki-Whan (2008-09-18). "Unusually Stable, Versatile, and Pure Arenediazonium Tosylates: Their Preparation, Structures, and Synthetic Applicability". Organic Letters. 10 (18): 3961–3964. doi:10.1021/ol8013528. ISSN 1523-7060. PMID 18722457.
^ ab "UK CRHF Incident Report - Supersaturated Diazonium salt causes Fatality". Retrieved 13 May 2010.
^ R. V. Hoffman (1981). "m-Trifluoromethylbenzenesulfonyl Chloride". Org. Synth. 60: 121. doi:10.15227/orgsyn.060.0121.
^ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
^ ab Klaus Hunger, Peter Mischke, Wolfgang Rieper, et al. "Azo Dyes" in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_245.
^ ab Clark, Jim. "chemguide". Retrieved 28 September 2011.
^ March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York.
ISBN 978-0-471-60180-7.
^ Marye Anne Fox; James K. Whitesell (2004). Organic Chemistry (3, illustrated ed.). Jones & Bartlett Learning. pp. 535–538. ISBN 978-0-7637-2197-8.
^ L. Gattermann (1894). "Untersuchungen über Diazoverbindungen". Berichte der Deutschen Chemischen Gesellschaft. 23 (1): 1218–1228. doi:10.1002/cber.189002301199.
^ Flood, D. T. (1933). "Fluorobenzene". Org. Synth. 13: 46. doi:10.15227/orgsyn.013.0046..
^ ab Kazem-Rostami, Masoud (2017). "Facile Preparation of Phenol". Synlett. 28 (13): 1641–1645. doi:10.1055/s-0036-1588180.
^ Carey, F. A.; Sundberg, R. J. (2007). Advanced Organic Chemistry. Vol. B, Chapter 11: Springer. p. 1028.CS1 maint: Multiple names: authors list (link)
^ Khazaei, Ardeshir; Kazem-Rostami, Masoud; Zare, Abdolkarim; Moosavi-Zare, Ahmad Reza; Sadeghpour, Mahdieh; Afkhami, Abbas (2013). "Diazonium to phenol". J. Appl. Polym. Sci. 129 (6): 3439–3446. doi:10.1002/app.39069.
^ Wu, Jie; Gao, Yueqiu; Qiu, Guanyinsheng; He, Linman (2014-08-20). "Removal of amino groups from anilines through diazonium salt-based reactions". Organic & Biomolecular Chemistry. 12 (36): 6965–6971. doi:10.1039/C4OB01286K. ISSN 1477-0539.
^ Sutton, D (1993). "Organometallic Diazo Compounds". Chem. Rev. 93 (3): 905–1022. doi:10.1021/cr00019a008.
^ Pinacho Crisóstomo Fernando (2014). "Ascorbic Acid as an Initiator for the Direct C-H Arylation of (Hetero)arenes with Anilines Nitrosated In Situ". Angewandte Chemie International Edition. 53 (8): 2181–2185. doi:10.1002/anie.201309761. PMID 24453180.
^ Green Chemical Functionalization of Single-Walled Carbon Nanotubes in Ionic Liquids B. Katherine Price, Jared L. Hudson, and James M. Tour J. Am. Chem. Soc.; 2005; 127(42) pp. 14867–14870. doi:10.1021/ja053998c
^ Michael P. Stewart; Francisco Maya; Dmitry V. Kosynkin; et al. (2004). "Direct Covalent Grafting of Conjugated Molecules onto Si, GaAs, and Pd Surfaces from Aryldiazonium Salts". J. Am. Chem. Soc. 126 (1): 370–8. doi:10.1021/ja0383120. PMID 14709104.
^ Reaction sequence: silicon surface reaction with ammonium hydrogen fluoride creates hydride layer. An electron is transferred from the silicon surface to the diazonium salt in an open circuit potential reduction leaving a silicon radical cation and a diazonium radical. In the next step a proton and a nitrogen molecule are expelled and the two radical residues recombine creating a surface silicon to carbon bond.
^ S.Q. Lud; M. Steenackers; P. Bruno; et al. (2006). "Chemical Grafting of Biphenyl Self-Assembled Monolayers on Ultrananocrystalline Diamond". J. Am. Chem. Soc. 128 (51): 16884–91. doi:10.1021/ja0657049. PMID 17177439.
^ De-en Jiang; Bobby G. Sumpter; Sheng Dai (2006). "Structure and Bonding between an Aryl Group and Metal Surfaces". J. Am. Chem. Soc. 128 (18): 6030–1. doi:10.1021/ja061439f. PMID 16669660.
External links
W. Reusch. "Reactions of Amines". VirtualText of Organic Chemistry. Michigan State University. Archived from the original on 2012-12-12.