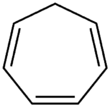
Cycloheptatriene

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
| |||
| Names | |||
|---|---|---|---|
Preferred IUPAC name Cyclohepta-1,3,5-triene[1] | |||
| Other names 1,3,5-Cycloheptatriene 1H-[7]Annulene CHT Tropilidene | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
Beilstein Reference | 506066 | ||
ChEBI |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.008.061 | ||
EC Number | 208-866-3 | ||
Gmelin Reference | 1943 | ||
PubChem CID |
| ||
UN number | 2603 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C7H8 | ||
Molar mass | 7001921410000000000♠92.141 g·mol−1 | ||
Density | 0.888 g/mL at 25 °C | ||
Melting point | −80 °C (−112 °F; 193 K) | ||
Boiling point | 116 °C (241 °F; 389 K) | ||
Solubility in water | Insoluble in water | ||
| Hazards | |||
GHS pictograms |     | ||
GHS signal word | Danger | ||
GHS hazard statements | H225, H301, H304, H311, H315, H319, H335 | ||
GHS precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+310, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P322, P330, P331, P332+313, P337+313, P361, P362 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and as a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium is.
Contents
1 Synthesis
2 Reactions
3 See also
4 References
5 External links
Synthesis
Albert Ladenburg first generated cycloheptatriene in 1881 by the decomposition of tropine.[2][3] The structure was finally proven by the synthesis of Richard Willstätter in 1901. This synthesis started from cycloheptanone and established the seven membered ring structure of the compound.[4]
Cycloheptatriene can be obtained in the laboratory by photochemical reaction of benzene with diazomethane or the pyrolysis of the adduct of cyclohexene and dichlorocarbene.[5] A related classic synthesis for a cycloheptatriene derivatives, the Buchner ring enlargement, starts with the reaction of benzene with ethyl diazoacetate to give the corresponding norcaradiene carboxylic acid, which at high temperatures rearranges with ring expansion to the cycloheptatriene carboxylic acid ethyl ester.[6][7]
Reactions
Removal of a hydride ion from the methylene bridge gives the planar and aromatic cycloheptatriene cation, also called the tropylium ion. A practical route to this cation employs PCl5 as the oxidant.[8] CHT behaves as a diene in Diels–Alder reactions. Many metal complexes of cycloheptatriene are known, including Cr(CO)3(C7H8)
[9] and cycloheptatrienemolybdenum tricarbonyl.[10]
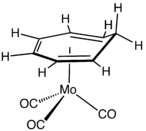
Structure of Mo(C7H8)(CO)3.
Cyclooctatetraene and cycloheptatriene are used as a triplet quencher for rhodamine 6G dye lasers.[11][12]
See also
- Cyclopentadiene
- Cyclooctatetraene
- Benzene
References
^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 223. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ A. Ladenburg (1883). "Die Constitution des Atropins". Justus Liebig's Annalen der Chemie. 217 (1): 74–149. doi:10.1002/jlac.18832170107.
^ A. Ladenburg (1881). "Die Zerlegung des Tropines". Berichte der Deutschen Chemischen Gesellschaft. 14 (2): 2126–2131. doi:10.1002/cber.188101402127.
^ R. Willstätter (1901). "Synthesen in der Tropingruppe. I. Synthese des Tropilidens". Justus Liebig's Annalen der Chemie. 317 (2): 204–265. doi:10.1002/jlac.19013170206.
^ H.E. Winberg (1959). "Synthesis of Cycloheptatriene". Journal of Organic Chemistry. 24 (2): 264–265. doi:10.1021/jo01084a635.
^ Buchner, et al., Ber., 18, 2377 (1885);
^ For a variation: Irvin Smith Lee; Tawney Pliny O (1934). "Studies on the Polymethylbenzenes. IX. Addition of Ethyl Diazoacetate to Durene". J. Am. Chem. Soc. 56 (10): 2167–2169. doi:10.1021/ja01325a054.
^ Conrow, K. (1973). "Tropylium Fluoroborate" (PDF). Organic Syntheses, Collected. 5: 1138.
^ James H. Rigby, Kevin R. Fales (2004). "7α-ACETOXY-(1Hβ, 6Hβ)-BICYCLO[4.4.1]UNDECA-2,4,8-TRIENE VIA CHROMIUM-MEDIATED HIGHER ORDER CYCLOADDITION". Organic Syntheses.; Collective Volume, 10, p. 1
^ Green, Malcolm L. H.; Ng, Dennis K. P. "Cycloheptatriene and -enyl Complexes of the Early Transition Metals" Chemical Reviews 1995, volume 95, pp. 439-73. doi:10.1021/cr00034a006
^ Tomi Nath Das; K. Indira Priyadarsini (1994). "Triplet of Cyclooctatetraene : Reactivity and Properties". Journal of the Chemical Society, Faraday Transactions. 90 (7): 963–968. doi:10.1039/ft9949000963.
^ R. Pappalardo; H. Samelson; A. Lempicki (1970). "Long Pulse Laser Emission From Rhodamine 6G Using Cyclooctatetraene". Applied Physics Letters. 16 (7): 267–269. doi:10.1063/1.1653190.