Aryl

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP

A phenyl group is the simplest aryl group, here bonded to an "R" group.
In the context of organic molecules, aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl.[1] "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams.
A simple aryl group is phenyl (with the chemical formula C6H5), a group derived from benzene. Examples of other aryl groups consist of:
- The tolyl group, CH3C6H4, which is derived from toluene (methylbenzene)
- The xylyl group, (CH3)2C6H3, which is derived from xylene (dimethylbenzene)
- The naphthyl group, C10H8, which is derived from naphthalene
Arylation is a chemical process in which an aryl group is attached to a substituent.
Contents
1 Nomenclature
2 Reactions
2.1 Electrophilic aromatic substitution
2.2 Halogen-metal exchange of aryl halides
3 See also
4 References
5 External links
Nomenclature
The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted for some substituent, and has the molecular formula C6H5−. Note that phenyl groups are not the same as benzyl groups, which consists of a phenyl group attached to a methyl group, and has the molecular formula C6H5CH2−.[2]

Phenol (or hydroxybenzene)
To name compounds containing phenyl groups, the phenyl group can be taken to be the parent hydrocarbon and being represented by the suffix "-benzene". Alternatively, the phenyl group could be treated as the substituent, being described within the name as "phenyl". This is usually done when the group attached to the phenyl group consists of six or more carbon atoms.[3]
As an example, consider a hydroxyl group connected to a phenyl group. In this case, if the phenyl group was taken as the parent hydrocarbon, the compound would be named hydroxybenzene. Alternatively, and more commonly, the hydroxyl group could be taken as the parent group (and the phenyl group treated as the substituent), resulting in the more familiar name phenol.
Reactions
Electrophilic aromatic substitution
Benzene rings have a delocalised pi-system of electrons, which creates areas of high negative charge. This makes aromatic compounds more prone to attacks by electrophilic reagents. However, due to the high stability of benzene rings, they will only react with highly reactive electrophiles, and will only undergo substitution reactions (but not addition reactions). Benzene's unusual stability is explained by its ability to delocalise charges by resonance. Electrophilic aromatic substitution of benzene takes place in two main steps: electrophilic attack and proton loss. The image below summarizes the general mechanism of an electrophilic aromatic substitution reaction.

An example of such reaction is between bromine and benzene. In this reaction, a bromine atom will substitute a hydrogen atom in the benzene ring, giving bromobenzene, an aryl halide. However, due to the unreactive nature of benzene, catalyst such as aluminium chloride is needed.[4] The formula for this reaction is:
- C6H6 + 0.5 Br2 → C6H5Br
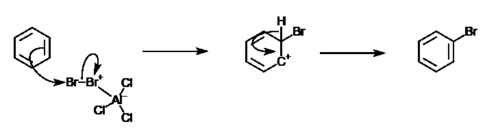
Halogen-metal exchange of aryl halides
Organometallic compounds are compounds that have a bond between a carbon and a metal atom.
The halogen atom of an aryl halide atom could be exchanged for a metal atom using an organometallic reagent. An example of such reaction is the reaction between bromobenzene and an organolithium reagent, where there is a nucleophilic attack of the lithium cation on bromine. The formula to such reaction is:
- C6H5Br + C4H10Li → C6H5Li + C4H10Br

The reaction is able to occur since benzene has a higher acidity compared to butane, which can be explained by the stability of the carbanion after the molecule loses a proton. The lithium-phenyl complex (C6H5Li) can better delocalize the negative charge by resonating it around the benzene ring; whereas in a lithium-butyl complex (C4H10Li), the molecule is not capable of such resonance and is further destabilized by the fact that the negative charge is placed on a primary carbon.[4] Hence, formation of the lithium-phenyl complex can be observed.
See also
- Alkyl
Aryl hydrocarbon receptor, a bodily target for dioxins[5]- Arene compound
References
^ IUPAC (2014). "aryl groups". In McNaught, A. D.; Wilkinson, A. Compendium of Chemical Terminology (2nd ed.). Oxford: Blackwell Scientific Publications. doi:10.1351/goldbook.A00464. ISBN 0-9678550-9-8..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Carey, Francis; Sundberg, Richard (2008). Advanced Organic Chemistry, Part A: Structure and Mechanisms (5th ed.). Springer.
^ IUPAC, Commission on Nomenclature of Organic Chemistry (1993). A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993). Blackwell Scientific Publications – via acdlabs.com.
^ ab Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry, 2nd Edition. Oxford University Press. ISBN 978-0-19-927029-3.
^ Bock KW, Köhle C (2006). "Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions". Biochem. Pharmacol. 72 (4): 393–404. doi:10.1016/j.bcp.2006.01.017. PMID 16545780.
External links
 Media related to Aryl groups at Wikimedia Commons
Media related to Aryl groups at Wikimedia Commons