Marmesin

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
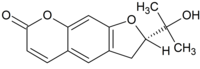 | |
| Names | |
|---|---|
IUPAC name (2S)-2-(2-Hydroxypropan-2-yl)-2,3-dihydrofuro[3,2-g]chromen-7-one | |
| Other names Nodakenetin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEMBL |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C14H14O4 |
Molar mass | 7002246262000000000♠246.262 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Marmesin (nodakenetin) is a chemical compound precursor in psoralen and linear furanocoumarins biosynthesis.[1]
References
^ Steck, Warren; Brown, Stewart A. (1971). "Comparison of (+)- and (−)-Marmesin as Intermediates in the Biosynthesis of Linear Furanoconmarins". Biochemistry and Cell Biology. 49 (11): 1213–1216. doi:10.1139/o71-174. ISSN 1208-6002..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
External links
Abu-Mustafa, Effat A.; Fayez, M. B. E. (1961). "Natural Coumarins. I. Marmesin and Marmesinin, Further Products from the Fruits of Ammi majus L.". The Journal of Organic Chemistry. 26 (1): 161–166. doi:10.1021/jo01060a039. ISSN 0022-3263.
This biochemistry article is a stub. You can help Wikipedia by expanding it. |