Phytochemistry

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP
| Part of a series on |
| Biochemistry |
|---|
 |
Key components |
|
|
History and topics |
|
|
|
|
Glossaries |
|
Portals: Biology, MCB |
Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Those studying phytochemistry strive to describe the structures of the large number of secondary metabolic compounds found in plants, the functions of these compounds in human and plant biology, and the biosynthesis of these compounds. Plants synthesize phytochemicals for many reasons, including to protect themselves against insect attacks and plant diseases. Phytochemicals in food plants are often active in human biology, and in many cases have health benefits.[1] The compounds found in plants are of many kinds, but most are in four major biochemical classes, the alkaloids, glycosides, polyphenols, and terpenes.
Phytochemistry can be considered sub-fields of botany or chemistry. Activities can be led in botanical gardens or in the wild with the aid of ethnobotany. The applications of the discipline can be for pharmacognosy, or the discovery of new drugs, or as an aid for plant physiology studies.
Contents
1 Techniques
2 Constituent elements
3 Eastern medicine
4 Phytochemicals
4.1 Alkaloids
4.2 Glycosides
4.3 Polyphenols
4.4 Terpenes
5 Major research institutes
6 References
7 Literature
Techniques
Techniques commonly used in the field of phytochemistry are extraction, isolation, and structural elucidation (MS,1D and 2D NMR) of natural products, as well as various chromatography techniques (MPLC, HPLC, and LC-MS).
Constituent elements
The list of simple elements of which plants are primarily constructed—carbon, oxygen, hydrogen, calcium, phosphorus, etc.—is not different from similar lists for animals, fungi, or even bacteria. The fundamental atomic components of plants are the same as for all life; only the details of the way in which they are assembled differs.
Eastern medicine
Phytochemistry is widely used in the field of Chinese medicine especially in the field of herbal medicine.
Phytochemical technique mainly applies to the quality control of Chinese medicine, Ayurvedic medicine(Indian traditional medicine) or herbal medicine of various chemical components, such as saponins, alkaloids, volatile oils, flavonoids and anthraquinones. In the development of rapid and reproducible analytical techniques, the combination of HPLC with different detectors, such as diode array detector (DAD), refractive index detector (RID), evaporative light scattering detector (ELSD) and mass spectrometric detector (MSD), has been widely developed.
In most cases, biologically active compounds in Chinese medicine, Ayurveda, or herbal medicine have not been determined.[citation needed] Therefore, it is important to use the phytochemical methods to screen and analyze bioactive components, not only for the quality control of crude drugs, but also for the elucidation of their therapeutic mechanisms. Modern pharmacological studies indicate that binding to receptors or ion channels on cell membranes is the first step of some drug actions. A new method in phytochemistry called biochromatography has been developed. This method combines human red cell membrane extraction and high performance liquid chromatography to screen potential active components in Chinese medicine.
Phytochemicals
Many plants produce chemical compounds for defence against herbivores. These are often useful as drugs, and the content and known pharmacological activity of these substances in medicinal plants is the scientific basis for their use. The major classes of pharmacologically active phytochemicals are described below, with examples of medicinal plants that contain them.[2] Human settlements are often surrounded by weeds useful as medicines, such as nettle, dandelion and chickweed.[3][4][5]
Many phytochemicals, including curcumin, epigallocatechin gallate, genistein and resveratrol are pan-assay interference compounds and are not useful in drug discovery.[6][7]
Alkaloids
Alkaloids are bitter-tasting chemicals, very widespread in nature, and often toxic. There are several classes with different modes of action as drugs, both recreational and pharmaceutical. Medicines of different classes include atropine, scopolamine, and hyoscyamine (all from nightshade),[8] the traditional medicine berberine (from plants such as Berberis and Mahonia),[a]caffeine (Coffea), cocaine (Coca), ephedrine (Ephedra), morphine (opium poppy), nicotine (tobacco),[b]reserpine (Rauwolfia serpentina), quinidine and quinine (Cinchona), vincamine (Vinca minor), and vincristine (Catharanthus roseus).[5][11]

The opium poppy Papaver somniferum is the source of the alkaloids morphine and codeine.[5]

The alkaloid nicotine from tobacco binds directly to the body's Nicotinic acetylcholine receptors, accounting for its pharmacological effects.[12]

Deadly nightshade, Atropa belladonna, yields tropane alkaloids including atropine, scopolamine and hyoscyamine.[8]
Glycosides
Anthraquinone glycosides are found in the laxatives senna,[13]rhubarb[14] and Aloe.[5]
The cardiac glycosides are powerful drugs from plants including foxglove and lily of the valley. They include digoxin and digitoxin which support the beating of the heart, and act as diuretics.[15]

Senna alexandrina, containing anthraquinone glycosides, has been used as a laxative for millennia.[13]

The foxglove, Digitalis purpurea, contains digoxin, a cardiac glycoside. The plant was used to treat heart conditions long before the glycoside was identified.[15][16]
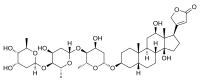
Digoxin is used to treat atrial fibrillation, atrial flutter and sometimes heart failure.[15]
Polyphenols
Polyphenols of several classes are widespread in plants. They include the colourful anthocyanins, hormone-mimicking phytoestrogens, and astringent tannins.[17][5] In Ayurveda, the astringent rind of the pomegranate is used as a medicine,[18] while polyphenol extracts from plant materials such as grape seeds are sold for their potential health benefits They have been continually studied in cell cultures for their different anti-cancer effects.[19][20]
Plants containing phytoestrogens have been used for centuries to treat gynaecological disorders such as fertility, menstrual, and menopausal problems.[21] Among these plants are Pueraria mirifica,[22]kudzu,[23]angelica,[24]fennel, and anise.[25]

Angelica, containing phytoestrogens, has long been used to treat gynaecological disorders.
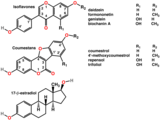
Polyphenols include phytoestrogens (top and middle), effective mimics of animal estrogen (bottom).[26]
Terpenes
Terpenes and terpenoids of many kinds are found in resinous plants such as the conifers. They are strongly aromatic and serve to repel herbivores. Their scent makes them useful in essential oils, whether for perfumes such as rose and lavender, or for aromatherapy.[5][27][28] Some have had medicinal uses: thymol is an antiseptic and was once used as a vermifuge (anti-worm medicine).[29]

The essential oil of common thyme (Thymus vulgaris), contains the monoterpene thymol, an antiseptic and antifungal.[29]
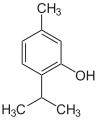
Thymol is one of many terpenes found in plants.[29]
Major research institutes
- Tropical Botanical Garden and Research Institute
- UBC Botanical Garden and Centre for Plant Research
References
^ John T. Arnason; Rachel Mata; John T. Romeo (2013-11-11). "Phytochemistry of Medicinal Plants". Springer Science & Business Media. ISBN 9781489917782..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ "Angiosperms: Division Magnoliophyta: General Features". Encyclopædia Britannica (volume 13, 15th edition). 1993. p. 609.
^ Meskin, Mark S. (2002). Phytochemicals in Nutrition and Health. CRC Press. p. 123. ISBN 978-1-58716-083-7.
^ Springbob, Karen & Kutchan, Toni M. (2009). "Introduction to the different classes of natural products". In Lanzotti, Virginia. Plant-Derived Natural Products: Synthesis, Function, and Application. Springer. p. 3. ISBN 978-0-387-85497-7.CS1 maint: Uses authors parameter (link)
^ abcdef Elumalai, A.; Eswariah, M. Chinna (2012). "Herbalism - A Review" (PDF). International Journal of Phytotherapy. 2 (2): 96–105.
^ Baell, Jonathan; Walters, Michael A. (24 September 2014). "Chemistry: Chemical con artists foil drug discovery". Nature. 513 (7519): 481–483. Bibcode:2014Natur.513..481B. doi:10.1038/513481a. PMID 25254460.
^ Dahlin JL, Walters MA (July 2014). "The essential roles of chemistry in high-throughput screening triage". Future Medicinal Chemistry. 6 (11): 1265–90. doi:10.4155/fmc.14.60. PMC 4465542. PMID 25163000.
^ ab "Atropa Belladonna" (PDF). The European Agency for the Evaluation of Medicinal Products. 1998. Retrieved 26 February 2017.
^ Yin, Jun; Xing, Huili; Ye, Jianping (May 2008). "Efficacy of Berberine in Patients with Type 2 Diabetes" (PDF). Metabolism. 57 (5): 712–717. doi:10.1016/j.metabol.2008.01.013. PMC 2410097. PMID 18442638.
^ Charlton, Anne (June 2004). "Medicinal uses of tobacco in history" (PDF). Journal of the Royal Society of Medicine. 97 (6): 292–296. doi:10.1258/jrsm.97.6.292. PMC 1079499. PMID 15173337.
^ Gremigni, P.; et al. (2003). "The interaction of phosphorus and potassium with seed alkaloid concentrations, yield and mineral content in narrow-leafed lupin (Lupinus angustifolius L.)". Plant and Soil. Heidelberg: Springer. 253 (2): 413–427. JSTOR 24121197.CS1 maint: Explicit use of et al. (link)
^ "Nicotinic acetylcholine receptors: Introduction". IUPHAR Database. International Union of Basic and Clinical Pharmacology. Retrieved 26 February 2017.
^ ab Hietala, P.; Marvola, M.; Parviainen, T.; Lainonen, H. (August 1987). "Laxative potency and acute toxicity of some anthraquinone derivatives, senna extracts and fractions of senna extracts". Pharmacology & toxicology. 61 (2): 153–6. doi:10.1111/j.1600-0773.1987.tb01794.x. PMID 3671329.
^ praful akolkar. "Pharmacognosy of Rhubarb". PharmaXChange.info.
^ abc "Active Plant Ingredients Used for Medicinal Purposes". United States Department of Agriculture. Retrieved 18 February 2017.
^ "Digitalis purpurea. Cardiac Glycoside". Texas A&M University. Retrieved 26 February 2017.The man credited with the introduction of digitalis into the practice of medicine was William Withering.
^ Da Silva, Cecilia; et al. (2013). "The High Polyphenol Content of Grapevine Cultivar Tannat Berries Is Conferred Primarily by Genes That Are Not Shared with the Reference Genome". The Plant Cell. Rockville, MD: American Society of Plant Biologists. 25 (12): 4777–4788. doi:10.1105/tpc.113.118810. JSTOR 43190600. PMC 3903987.CS1 maint: Explicit use of et al. (link)
^ K. K. Jindal; R. C. Sharma (2004). Recent trends in horticulture in the Himalayas. Indus Publishing. ISBN 81-7387-162-0.
^ Halliwell, B. (2007). "Dietary polyphenols: Good, bad, or indifferent for your health?". Cardiovascular Research. 73 (2): 341–347. doi:10.1016/j.cardiores.2006.10.004. PMID 17141749.
^ European Food Safety Authority (2010). "Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061". EFSA Journal. 8 (2): 1489. doi:10.2903/j.efsa.2010.1489.
^ Muller-Schwarze D (2006). Chemical Ecology of Vertebrates. Cambridge University Press. p. 287. ISBN 978-0-521-36377-8.
^ Lee YS, Park JS, Cho SD, Son JK, Cherdshewasart W, Kang KS (Dec 2002). "Requirement of metabolic activation for estrogenic activity of Pueraria mirifica". Journal of Veterinary Science. 3 (4): 273–277. PMID 12819377. Archived from the original on 2008-11-20.
^ Delmonte P, Rader JI (2006). "Analysis of isoflavones in foods and dietary supplements". Journal of AOAC International. 89 (4): 1138–46. PMID 16915857.
^ Brown D, Walton N (1999). Chemicals from Plants: Perspectives on Plant Secondary Products. World Scientific Publishing. pp. 21, 141. ISBN 978-981-02-2773-9.
^ Albert-Puleo M (Dec 1980). "Fennel and anise as estrogenic agents". Journal of Ethnopharmacology. 2 (4): 337–44. doi:10.1016/S0378-8741(80)81015-4. PMID 6999244.
^ Turner, J.V.; Agatonovic-Kustrin, S.; Glass, B.D. (Aug 2007). "Molecular aspects of phytoestrogen selective binding at estrogen receptors". Journal of Pharmaceutical Sciences. 96 (8): 1879–85. doi:10.1002/jps.20987. PMID 17518366.CS1 maint: Multiple names: authors list (link)
^ Tchen, T. T. (1965). "Reviewed Work: The Biosynthesis of Steroids, Terpenes & Acetogenins". American Scientist. Research Triangle Park, NC: Sigma Xi, The Scientific Research Society. 53 (4): 499A–500A. JSTOR 27836252.
^ Singsaas, Eric L. (2000). "Terpenes and the Thermotolerance of Photosynthesis". New Phytologist. New York: Wiley. 146 (1): 1–2. doi:10.1046/j.1469-8137.2000.00626.x. JSTOR 2588737.
^ abc "Thymol (CID=6989)". NIH. Retrieved 26 February 2017.THYMOL is a phenol obtained from thyme oil or other volatile oils used as a stabilizer in pharmaceutical preparations, and as an antiseptic (antibacterial or antifungal) agent. It was formerly used as a vermifuge.
Literature
.mw-parser-output .refbeginfont-size:90%;margin-bottom:0.5em.mw-parser-output .refbegin-hanging-indents>ullist-style-type:none;margin-left:0.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>ddmargin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none.mw-parser-output .refbegin-100font-size:100%
^ Berberine is the main active component of an ancient Chinese herb Coptis chinensis French, which has been used to attempt to treat diabetes for thousands of years, although there is no sound evidence of efficacy.[9]
^ Nicotine has "probably been responsible for more deaths than any other herb", but it was used as a medicine in the societies encountered by Columbus and was considered a panacea in Europe, although it is no longer accepted as medicinal.[10]
Girish Dwivedi; Dwivedi Shridhar (2007). "History of Medicine: Sushruta – the Clinician – Teacher par Excellence" (PDF). Indian J Chest Dis Allied Sc. 49: 243–244.









